Targeting chemokine receptor CXCR7
Cancer is the second leading cause of death in the US and afflicts over 13 million Americans. With approximately 1,600,000 new cancer diagnoses anticipated in 2013, it is clear that cancer imposes an immense burden on public health. This proposal focuses on the development of novel therapeutic agents to selectively target and destroy leukemia, glioblastoma multiforme (GBM), and breast cancer cells, as well as tumor-associated blood vessels, and thus has the potential to substantially improve public health.
CXCR7 is an atypical chemokine receptor that plays a critical role in guiding progenitor cell migration during embryo- and organo-genesis. Following fetal development and birth, however, expression of CXCR7 protein is difficult to detect on cells or tissues, except in the context of cancer. Expression of CXCR7 is regulated by hypoxia-inducible factor 1, which is commonly induced in the low oxygen environment of solid tumors. In normal adult human tissue, CXCR7 RNA and protein is expressed in the kidney and brain, although in neurons the protein is found intracellular and not on the cell surface (the cellular localization of CXCR7 protein in parenchymal kidney cells or renal multipotent progenitor cells is not known). In the context of cancer, CXCR7 RNA and protein are expressed in leukemia/lymphoma cells, GBM, breast cancer (primary invasive ductal carcinoma), lung cancer, and solid tumor-associated blood vessels. CXCR7 protein alone is also detected in rhabdomyosarcoma and cervix squamous cell carcinoma. CXCR7 RNA alone is also expressed in pituitary adenomas, renal carcinoma, pancreatic adenocarcinoma, and oral squamous cell carcinoma. CXCR7 surface protein is largely restricted to cancer cells or tumor-associated vessels and is thus a suitable selective target for therapeutic intervention.
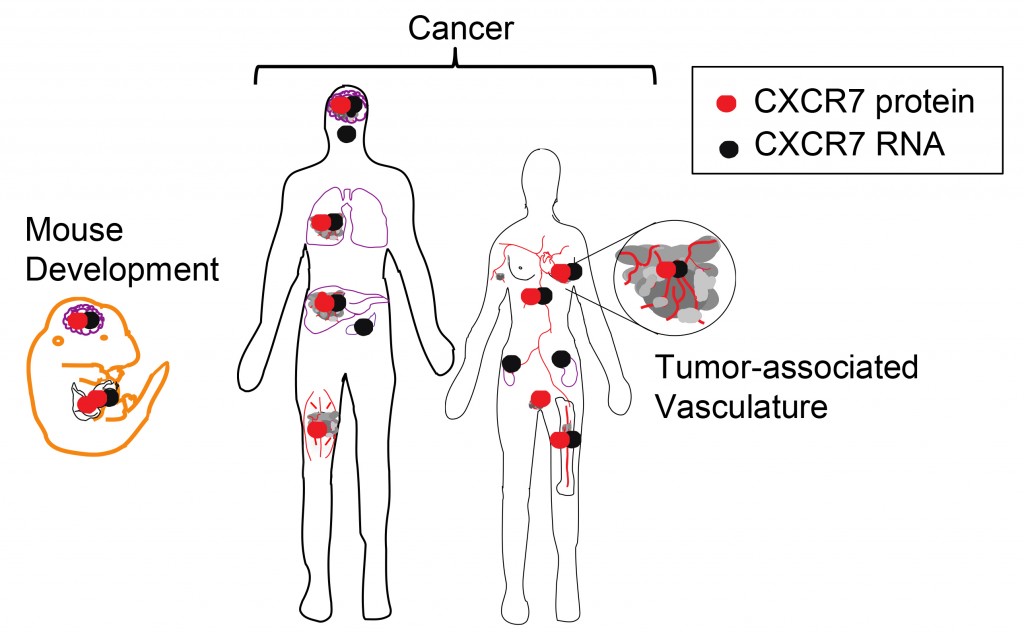
Graphical summary of CXCR7 RNA and protein expression in development and cancer. CXCR7 RNA (black symbols) and protein (red symbols) indicate anatomic sites of CXCR7 expression during fetal mouse development and in cancer. The protein expression summary is limited to those studies that used highly validated aCXCR7 mAb clone 11G8, as other antibody reagents may be non-specific. CXCR7 RNA and protein is expressed during mouse development of the liver and heart ; by primitive RBC; and during fetal neuronal migration. In cancer, CXCR7 RNA and protein is expressed in blood, brain, lung, and breast cancer, and by solid tumor-associated blood vessels. CXCR7 protein is also expressed in rhabdomyosarcoma and cervix squamous cell carcinoma, while CXCR7 RNA is also expressed in pituitary adenomas, renal carcinoma, pancreatic adenocarcinoma, and oral squamous cell carcinoma.
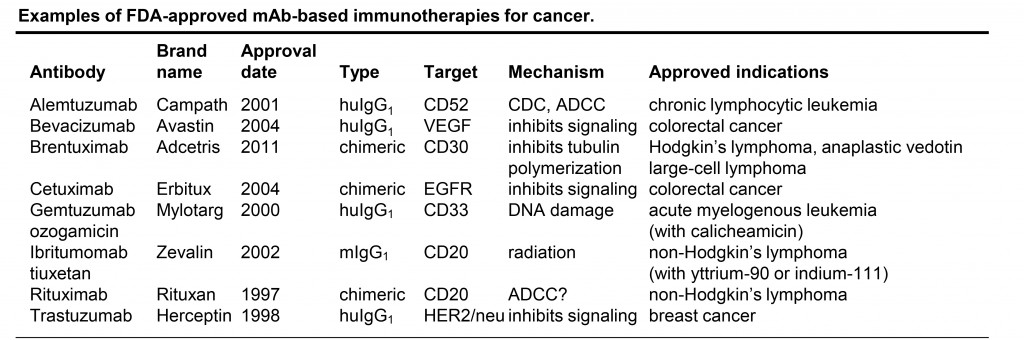
Monoclonal antibody-based cancer immunotherapy has emerged as a powerful and precise modality to effectively target and inhibit tumor cell growth and survival. Unconjugated mAb use a variety of mechanisms to kill tumor cells, including antibody-dependent cellular cytotoxicity (ADCC) mediated by natural killer (NK) cells and other immune effectors, or complement-dependent cytotoxicity (CDC). Receptor-targeted antibodies can also block ligand binding, or elicit receptor internalization, thus decreasing responsiveness of tumor cells to extracellular survival and proliferative signals. Campath, Avastin, Erbitux, Rituxan, and Herceptin are examples of approved unconjugated mAb-based immunotherapies (see Table).
Cytotoxic bacterial toxins such as PE38, a 38-kDa truncated derivative of Pseudomonas aeruginosa exotoxin A, are also used as warheads conjugated to mAbs for immunotherapy. This strategy relies on receptor/payload internalization, as PE38 functions to inhibit translation and is only active inside the cell. PE38-conjugated immunotoxins do not require specialized conjugation or handling procedures like those required for radioactive- or chemical-toxin-based immunotherapies, and do not require an intact immune system for in vivo efficacy like many unconjugated mAbs.
Another promising immunotherapy approach based on mAbs is bispecific T-cell engagers (BiTE). These are engineered tethered divalent proteins in which one mAb fragment variable (Fv) region binds to a T cell (typically targeting CD3) and the other Fv region binds to a tumor specific antigen. Typically, T cell activation requires recognition of cognate antigen presented by MHC, however, BiTEs act to circumvent this requirement by nonspecifically activating T cells via clustering surface CD3. The close proximity of the target tumor cells facilitated by Fv binding to tumor antigen enables directed tumor cell killing.
The overall objective of this project is to generate novel CXCR7-targeted mAb-based immunotherapeutic proteins and test their ability to reverse cancer progression.
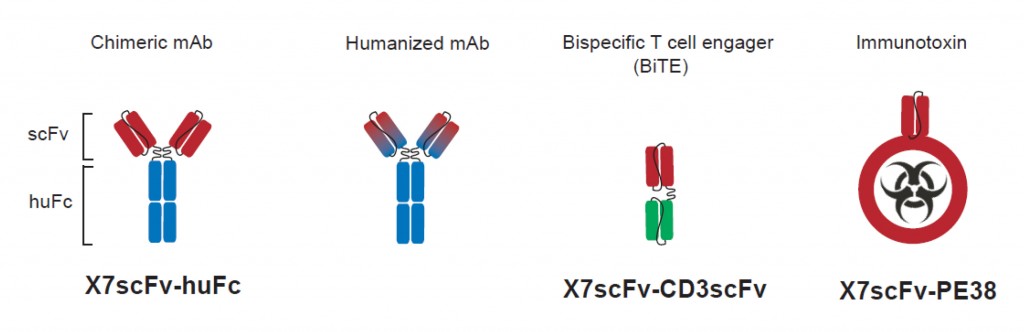
CXCR7-targeted cytolytic proteins. The Fv domains (VL and VH) of mouse aCXCR7 monoclonal antibody (red) were grafted onto a human IgG1 framework (blue) to generate the single chain CXCR7-targetd human FC chimeric antibody (X7scFv-huFC). Although encoded as a single chain monomer, like most scFv-FC constructs the chimeric protein is predicted to homodimerize resulting in a classical antibody structure (depicted above). The X7scFv-huFC will be further humanized by altering non-complementarity-determining regions in the Fv domains to make the fusion protein less immunogenic while retaining CXCR7 specificity. The X7scFv will be fused to CD3scFv to form a bispecific T cell engager (BiTE) as a complementary approach to target and destroy CXCR7+ tumor cells using cytotoxic T cells. The X7scFv will also be fused to Pseudomonas aeruginosa exotoxin A (PE38) to generate a CXCR7-targeted immunotoxin that, upon binding and internalization, will kill the target cell by inhibiting translation.
This project will create and test novel therapeutic agents to selectively target and destroy leukemia, GBM, and breast cancer cells, and, importantly, tumor-associated blood vessels. We believe it holds great promise to improve the health and welfare of cancer patients in the US.
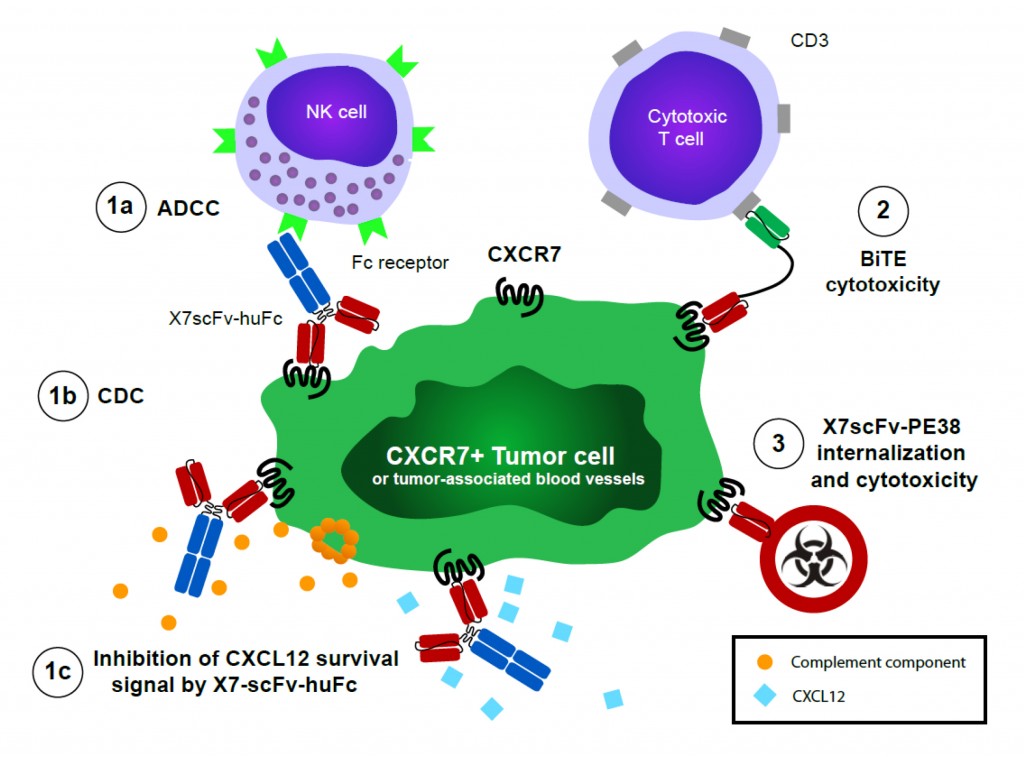
Killing CXCR7+ tumor cells with an array of novel cytolytic scFv fusion proteins . Chimeric X7scFv-huFC antibody utilizes human PBMC or mouse NK cells to kill CXCR7+ tumor cells by ADCC (1a). The chimeric antibody may also fix complement on the cell surface to kill by complement-dependent cytotoxicity (CDC)(1b). The chimeric antibody blocks CXCL12-dependent signaling, which may inhibit key chemokine-dependent survival signals or block metastatic spread by inhibiting transendothelial migration (1c). We hypothesize that a bispecific T cell engager (BiTE) comprised of X7scFv-CD3scFv (FC-domain optional) will serve as an ‘immunobridge’ to enable killing of CXCR7+ cells by cytolytic T cells (2). We further hypothesize that immunotoxin (PE38)-conjugated X7scFv will be internalized by CXCR7+ cells to enable selective and potent killing, which may be particularly useful for solid tumors refractory to other therapies (3). In addition, we hypothesize that the array of cytolytic fusion proteins will also target CXCR7+ endothelial cells selectively upregulated on tumor-feeding blood vessels, offering another tumor-associated target for reversing cancer progression.